Oncogenic Kras driven metabolic reprogramming in pancreas cancer cells utilizes cytokines from the tumor microenvironment.
Authors: Prasenjit Dey, Jun Li, Jianhua Zhang, Surendra Chaurasiya, Anders Strom, Huamin Wang, Wen-Ting Liao, Frederick Cavallaro, Parker Denz, Vincent P. Bernard, Er-Yen Yen, Giannicola Genovese, Prateek Gulhati, Jielin Liu, Deepavali Chakravarti, Pingna Deng, Tingxin Zhang, Federica Carbone, Edward Chang, Haoqiang Ying, Xiaoying Shang, Denise J. Spring, Bidyut Ghosh, Nagireddy Putluri, Anirban Maitra, Y. Alan Wang, and Ronald A. DePinho
Abstract:
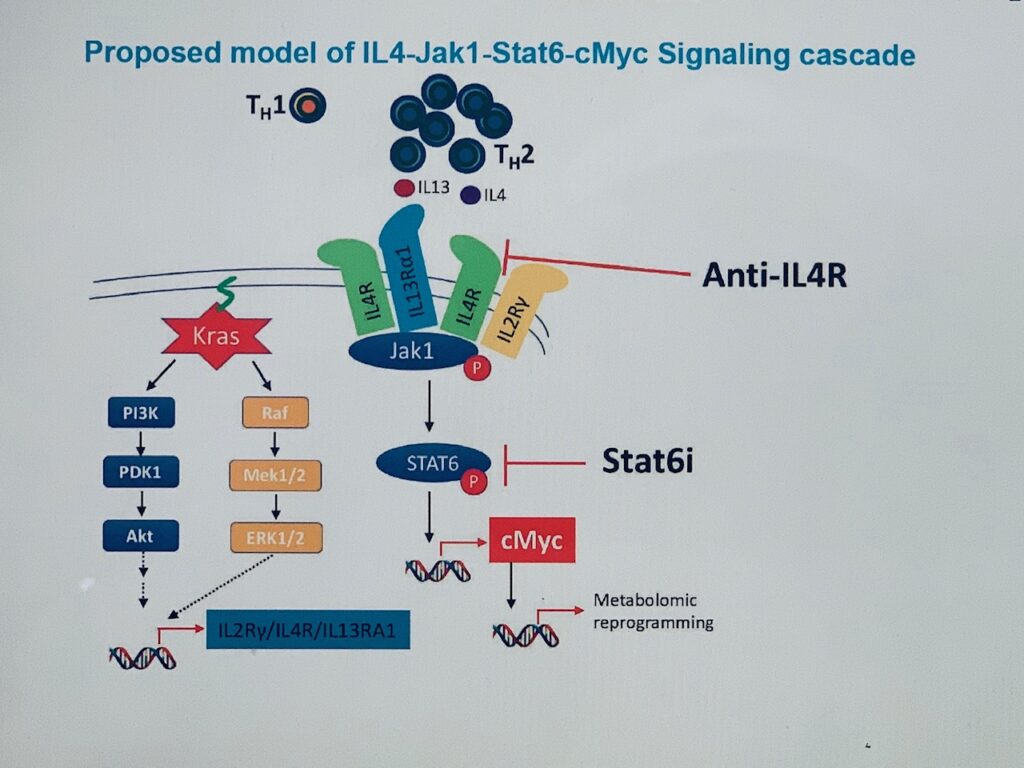
A hallmark of pancreatic ductal adenocarcinoma (PDAC) is an exuberant stroma comprised of diverse cell types that enable or suppress tumor progression. We explored the role of oncogenic Kras in pro-tumorigenic signaling interactions between cancer cells and host cells and show that Kras* drives cell autonomous expression of type I cytokine receptor complexes (IL2ry-IL4ra and IL2ry-IL13ra1) in cancer cells that in turn are capable of receiving cytokine growth signals (IL4 or IL13) provided by invading TH2 cells in the microenvironment. Early neoplastic lesions show close proximity of Kras* cancer cells and TH2 cells producing IL4 and IL13. Activated IL2ry-IL4ra and IL2ry-IL13ra1 receptors signal primarily via Jak1-Stat6. Integrated transcriptomic, chromatin occupancy and metabolomic studies identified cMyc, as a direct target of activated Stat6, and that cMyc drives glycolysis. Thus paracrine signaling in the tumor microenvironment plays a key role in the Kras*-driven metabolic reprogramming of PDAC.
Read full article: Cancer Discovery