Pat Gulhati1and Ronald DePinho2
1Department of Medical Oncology, Rutgers Cancer Institute of New Jersey, New Brunswick, New Jersey, USA. 1Department of Cancer Biology, MD Anderson Cancer Center, Houston, Texas, USA. Pat Gulhati1and Ronald DePinho2
Pancreatic cancer (PDAC) is one of the most lethal human cancers1. The mainstay of treatment for metastatic PDAC is chemotherapy, which offers modest and transient benefit. While immune checkpoint therapy (ICT) has transformed treatment for many advanced cancers, PDAC has been recalcitrant to ICT, including anti-PD1 and anti-CTLA42,3, and is considered refractory to immune therapy.
The presence of novel immune checkpoints on T cells in human and murine PDAC 2 prompted exploration of their functional significance as targets alone and in combination. In particular, the preponderance of myeloid cells in the tumor immune microenvironment (TIME) and correlation with poor outcomes, suggested their importance in facilitating PDAC growth and limiting the therapeutic effects of ICT2,4. The urgent need to identify novel therapeutic targets for PDAC, motivated to study the contribution of alternative immune checkpoints and/or cooperative immune suppressive mechanisms across the stroma and myeloid cells in the TIME.
The Discovery
At baseline, the TIME of human and murine PDAC is dominated by CXCR2-expressing myeloid derived suppressor cells (MDSCs) and tumor associated macrophages (TAMs), as well as CD4+ Tregs with high CTLA4 and OX40 expression and exhausted CD8+ T cells with high PD-1, LAG3, 41BB and TIM3 expression. We identified CD8+ T cells transition from naïve/central memory to activated/non-exhausted to an exhausted state in the TIME5. Expression of these inhibitory/activating immune checkpoints was detected on the exhausted CD8+ T cells, but not on the naïve/central memory or non-exhausted/activated CD8+ T cell states, suggesting that these molecules may mediate the exhausted state of CD8+ T cells in the TIME. The iKRAS model of PDAC (oncogenic KRAS, p53 null), which faithfully mirrors the human disease, was used to assess the therapeutic effects of targeting each of the immune targets on growth of large, established autochthonous and orthotopic PDAC tumors.
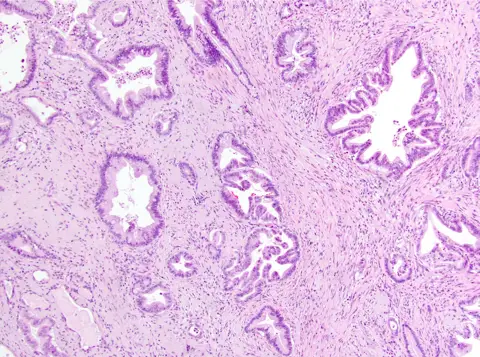
While antagonist PD1 and CTLA4 ICT agents are ineffective, mirroring responses in patients3, agonist 41BB and antagonist LAG3 ICT demonstrated efficacy alone and in combination, reprogramming the TIME towards anti-tumor immunity by modulating T cell subsets with anti-tumor activity, increasing T cell clonality and diversification, decreasing immunosuppressive myeloid cells and increasing antigen presentation/decreasing immunosuppressive capability of myeloid cells. Translational analyses confirmed 41BB and LAG3 expression on T cells in 81% and 93% of human PDAC specimens, respectively (Fig. 1a). As single and dual ICTs were not curative, these agents were combined with CXCR1/2 inhibitor, SX-682, targeting MDSCs. Triple therapy produced unprecedented durable disease eradication – 90% mice were cured of orthotopic iKRAS tumors, and there was three-fold improvement in survival and no evidence of disease in 20% mice with autochthonous iKRAS tumors upon necropsy following >6 months of treatment. Thus, anti-tumor immunity can be awakened in pancreas cancer.
The comprehensive profiling and rationale immune therapeutic testing in this study revealed, for the first time, that effective immune treatment is possible in pancreas cancer. The approach required neutralization of distinct reinforcing immunosuppressive mechanisms. These pancreas cancer findings raise the possibility that other ‘immunologically cold’ cancer types may be conquered by similar experimental approaches.
This work epitomizes the power of teamwork. It was made possible by a collaborative effort between groups with expertise in PDAC mouse models, human PDAC tumor specimens, tumor biology and immunotherapy, single-cell RNA sequencing, and computational biology.
1Department of Medical Oncology, Rutgers Cancer Institute of New Jersey, New Brunswick, New Jersey, USA. 1Department of Cancer Biology, MD Anderson Cancer Center, Houston, Texas, USA. Pat Gulhati1and Ronald DePinho2
Pancreatic cancer (PDAC) is one of the most lethal human cancers1. The mainstay of treatment for metastatic PDAC is chemotherapy, which offers modest and transient benefit. While immune checkpoint therapy (ICT) has transformed treatment for many advanced cancers, PDAC has been recalcitrant to ICT, including anti-PD1 and anti-CTLA42,3, and is considered refractory to immune therapy.
The presence of novel immune checkpoints on T cells in human and murine PDAC 2 prompted exploration of their functional significance as targets alone and in combination. In particular, the preponderance of myeloid cells in the tumor immune microenvironment (TIME) and correlation with poor outcomes, suggested their importance in facilitating PDAC growth and limiting the therapeutic effects of ICT2,4. The urgent need to identify novel therapeutic targets for PDAC, motivated to study the contribution of alternative immune checkpoints and/or cooperative immune suppressive mechanisms across the stroma and myeloid cells in the TIME.
The Discovery
At baseline, the TIME of human and murine PDAC is dominated by CXCR2-expressing myeloid derived suppressor cells (MDSCs) and tumor associated macrophages (TAMs), as well as CD4+ Tregs with high CTLA4 and OX40 expression and exhausted CD8+ T cells with high PD-1, LAG3, 41BB and TIM3 expression. We identified CD8+ T cells transition from naïve/central memory to activated/non-exhausted to an exhausted state in the TIME5. Expression of these inhibitory/activating immune checkpoints was detected on the exhausted CD8+ T cells, but not on the naïve/central memory or non-exhausted/activated CD8+ T cell states, suggesting that these molecules may mediate the exhausted state of CD8+ T cells in the TIME. The iKRAS model of PDAC (oncogenic KRAS, p53 null), which faithfully mirrors the human disease, was used to assess the therapeutic effects of targeting each of the immune targets on growth of large, established autochthonous and orthotopic PDAC tumors.
While antagonist PD1 and CTLA4 ICT agents are ineffective, mirroring responses in patients3, agonist 41BB and antagonist LAG3 ICT demonstrated efficacy alone and in combination, reprogramming the TIME towards anti-tumor immunity by modulating T cell subsets with anti-tumor activity, increasing T cell clonality and diversification, decreasing immunosuppressive myeloid cells and increasing antigen presentation/decreasing immunosuppressive capability of myeloid cells. Translational analyses confirmed 41BB and LAG3 expression on T cells in 81% and 93% of human PDAC specimens, respectively (Fig. 1a). As single and dual ICTs were not curative, these agents were combined with CXCR1/2 inhibitor, SX-682, targeting MDSCs. Triple therapy produced unprecedented durable disease eradication – 90% mice were cured of orthotopic iKRAS tumors, and there was three-fold improvement in survival and no evidence of disease in 20% mice with autochthonous iKRAS tumors upon necropsy following >6 months of treatment. Thus, anti-tumor immunity can be awakened in pancreas cancer.
The comprehensive profiling and rationale immune therapeutic testing in this study revealed, for the first time, that effective immune treatment is possible in pancreas cancer. The approach required neutralization of distinct reinforcing immunosuppressive mechanisms. These pancreas cancer findings raise the possibility that other ‘immunologically cold’ cancer types may be conquered by similar experimental approaches.
This work epitomizes the power of teamwork. It was made possible by a collaborative effort between groups with expertise in PDAC mouse models, human PDAC tumor specimens, tumor biology and immunotherapy, single-cell RNA sequencing, and computational biology.